FDA Clearance Importance
- ✓ Trust in Safety
- ✓ Clinical Validity
- ✓ Insurance Coverage

What if you could harness the power of light to heal without invasive procedures? Low-level laser therapy (LLLT) is emerging as a groundbreaking solution in the medical field. This article dives deep into the FDA regulations surrounding LLLT and its applications, offering crucial insights for both practitioners and patients.
Understanding the critical steps and implications of FDA clearance for Low-Level Laser Therapy (LLLT) devices.
When we talk about low-level laser therapies (LLLT), it’s essential to grasp not just what they are, but also how they fit into our healthcare system. As a passionate advocate for LLLT, I, Dr. Malcolm Kane, have seen firsthand the benefits of this non-invasive healing technology. But what makes it safe and effective? That’s where understanding FDA regulations comes into play.
Low-level laser therapy leverages specific wavelengths of light to promote healing and reduce pain. This approach is gaining traction in various clinical settings, thanks to its ability to enhance cellular function without the drawbacks of invasive procedures. As we delve deeper, you'll see how crucial it is for these therapies to have the right regulatory backing. For comprehensive information on medical lasers and their regulation, the FDA's official page on medical lasers is an invaluable resource.
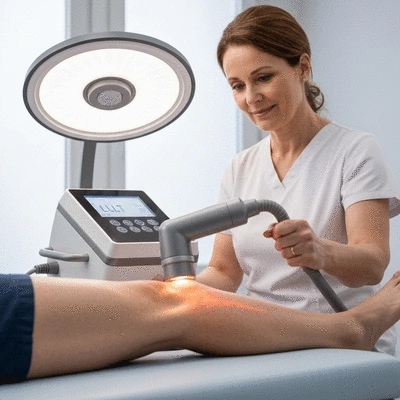
Low-level laser therapy (LLLT) refers to a therapeutic technique that uses low-power lasers to stimulate healing in tissues. This treatment is often employed for conditions like chronic pain, inflammation, and even wound healing. Here’s a quick overview of how LLLT works:
Understanding these fundamentals equips both patients and practitioners to appreciate the potential of LLLT in modern medical practice. Further details on laser product conformance can be found in FDA Laser Notice No. 56.
FDA clearance is a critical milestone for any medical device, including LLLT systems. It serves as a validation of safety and efficacy, ensuring that the technologies we use are not only effective but also safe for patients. FDA clearance means:
Thus, obtaining FDA clearance is not just a regulatory hurdle; it’s an essential step towards ensuring that LLLT can be widely and safely adopted in various healthcare settings.
LLLT has a broad range of applications in the medical field. By understanding these uses, we can better appreciate the significance of FDA regulations. Here’s a summary of where LLLT shines:
These applications highlight the versatility of LLLT and the importance of FDA oversight in ensuring that patients receive treatments that are both safe and effective. At Erchonia Laser, we are committed to championing the science behind LLLT, advocating for its rightful place in modern medicine. For more information on laser products and instruments, the FDA provides detailed guidelines.
Have you or someone you know ever tried low-level laser therapy (LLLT) for pain management or healing? We’d love to hear about your experiences!
LLLT is a therapeutic technique that uses low-power lasers to stimulate healing in tissues, reduce inflammation, and alleviate pain without invasive procedures. It works by enhancing cellular activity and circulation.
FDA clearance is crucial because it validates the safety and efficacy of LLLT devices. This ensures that the devices meet rigorous safety standards, are clinically validated, and can be eligible for insurance coverage, fostering trust among patients and practitioners.
LLLT is widely used for pain management (e.g., arthritis, tendonitis), accelerating wound healing by promoting tissue repair, and stimulating hair follicles for hair restoration.
Practitioners must ensure that LLLT devices are properly classified by the FDA, maintain rigorous documentation of clinical evidence, engage with regulatory affairs specialists, and stay updated on evolving FDA guidelines to ensure compliance and patient safety.
Valuable resources include the FDA Official Website for regulations and guidance, the Laser Institute of America for educational materials on laser safety, and the Medical Device Network for industry news and regulatory updates.
As we reflect on the journey through FDA regulations for low-level laser therapy (LLLT), it’s crucial for both practitioners and manufacturers to grasp the fundamental aspects of FDA clearances. Understanding these regulations not only aids in compliance but also ensures that effective therapies reach those in need. Here are some key considerations to keep in mind:
By being aware of these aspects, healthcare professionals can better navigate the regulatory landscape while ensuring patient safety and treatment efficacy. At Erchonia Laser, we are committed to empowering practitioners with the knowledge they need to make informed decisions regarding LLLT applications.

Practitioners and manufacturers must consider several factors when developing and utilizing LLLT devices. Here’s a concise list of essential considerations:
Incorporating these elements into your practice not only enhances credibility but also fosters trust among patients seeking non-invasive treatments. By prioritizing compliance, we can collectively elevate the standards of care in the field of laser therapy.
For those looking to expand their understanding of FDA regulations for LLLT, several resources can provide valuable insights. Here’s a list of recommended resources:
Utilizing these resources can significantly enhance your knowledge base and help ensure compliance. At Erchonia Laser, we encourage ongoing education and dialogue about the evolving landscape of laser therapy regulations.
Regulatory affairs play a critical role in the development and marketing of low-level laser therapy devices. This field ensures that products meet the necessary safety and efficacy standards before they reach consumers. Here are the key responsibilities within regulatory affairs:
By integrating regulatory affairs into the development process, companies can expedite the approval timeline while ensuring safety and quality. This proactive approach is essential to driving innovation in the LLLT field.
As we continue to navigate the complexities of FDA regulations for low-level laser therapy, it’s vital to remain informed and compliant. Join me in fostering a community that prioritizes patient safety and effective treatments. Here’s how you can stay engaged:
At Erchonia Laser, we believe in the power of shared knowledge. Join our community to receive the latest updates on regulatory changes impacting LLLT. Together, we can stay ahead of the curve and ensure our practices are aligned with the highest standards.
To assist you further in your regulatory journey, we’ve created a comprehensive checklist to help navigate FDA clearances. Download it today to streamline your compliance process and enhance your understanding of FDA requirements!
Here is a quick recap of the important points discussed in the article:
Healing Journeys with Erchonia Laser

Curious about how simple light can transform your pain management journey? Erchonia Laser Therapy of
Understanding Erchonia Laser Therapy Benefits
Have you ever considered a solution for chronic pain that doesn’t involve surgery or medication? E
LLLT and Improved Blood Flow
Healing Journeys with Erchonia Laser
Understanding Erchonia Laser Therapy Benefits
Erchonia Devices in Laser Therapy
Biphasic Response in Low-Level Laser