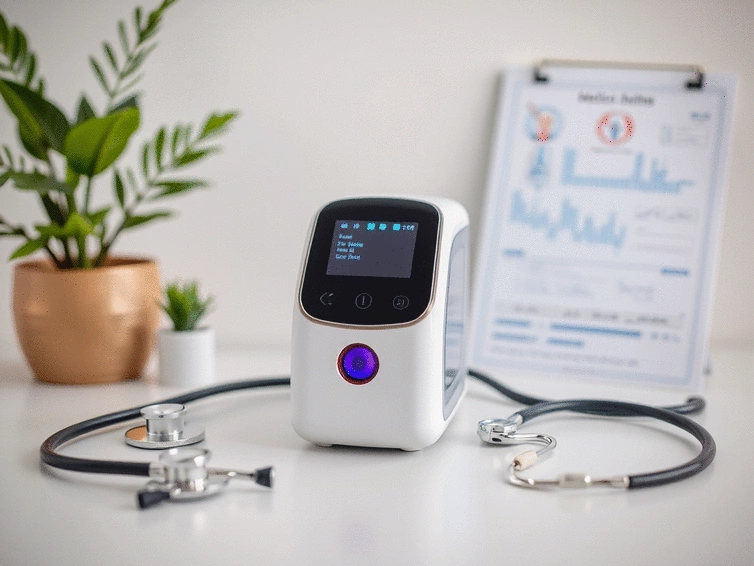
Class ILow-Risk
Minimal regulatory controls, lowest risk.
Have you ever considered the trustworthiness of the medical devices you use? Understanding FDA clearances can significantly bolster your confidence in low-level laser therapy (LLLT) devices, paving the way for informed treatment decisions. Let’s explore the core lessons that this article imparts about LLLT and its regulatory backdrop.
This visual outlines the FDA's classification of Low-Level Laser Therapy (LLLT) devices by risk level and the associated approval pathways.
Submission of data to demonstrate equivalence
Review of clinical evidence by the FDA
Clearance granted if device meets standards
Minimal regulatory controls, lowest risk.
Requires FDA 510(k) clearance.
Requires extensive pre-market approval.
As we dive into the world of low-level laser therapy (LLLT), it's essential to understand the importance of FDA clearances for these innovative devices. FDA clearance signifies that a product has met the necessary safety and efficacy standards, ensuring that patients can trust the technology used in their treatments. This article will explore what LLLT devices are, their various applications, and why FDA clearance plays a crucial role in the landscape of pain management and wellness.
Low-level laser therapy is a non-invasive treatment that utilizes specific wavelengths of light to stimulate cellular processes. This can lead to enhanced healing, reduced inflammation, and pain relief. By shining a light on how LLLT works, we can better appreciate its role in modern healthcare! Let’s jump into the various applications of these devices.
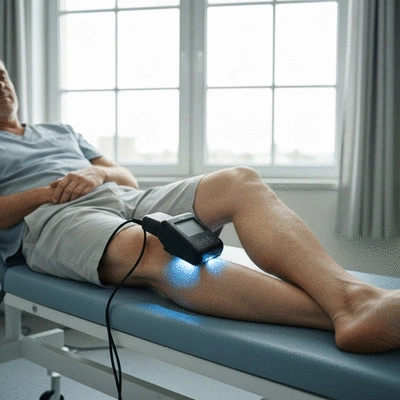
Low-level laser therapy (LLLT) refers to the use of low-intensity lasers or light-emitting diodes (LEDs) to promote healing without damaging tissues. Essentially, LLLT enhances cellular metabolism, which can aid in tissue repair and pain relief. It’s like giving a gentle nudge to the body’s natural healing processes!
The mechanism behind LLLT involves the absorption of light by cellular chromophores, which can boost ATP production and improve blood circulation. This leads to faster recovery times and improved quality of life for patients seeking non-invasive solutions.
LLLT devices are not just limited to pain management; they also have remarkable applications in aesthetic treatments and hair loss. Many practitioners, including those at Erchonia Laser, have reported success in using LLLT for:
These diverse applications underscore the versatility of LLLT and its growing popularity among healthcare professionals and patients alike! For additional guidance on LLLT devices for aesthetic purposes, you can refer to the FDA's guidance on low-level laser systems for aesthetic use.
In the realm of pain management, LLLT has gained attention as a powerful tool for treating various conditions. Its therapeutic uses span a wide range of issues, including:
Many patients have found relief through LLLT, often reporting significant improvements after just a few sessions. With ongoing research and clinical trials, the potential for LLLT in pain management continues to expand!
As I mentioned earlier, FDA clearance is vital for establishing trust in LLLT devices. It assures patients and practitioners alike that the devices have been rigorously tested for safety and efficacy. Let’s break down what FDA clearance entails and why it matters.
FDA clearance means the device has gone through extensive evaluation to confirm it meets safety and effectiveness standards. This process helps ensure that patients receive reliable and safe treatments. For LLLT devices, the FDA assesses:
This thorough examination process ensures that patients can trust the treatments they receive and encourages healthcare providers to integrate these devices into their practice.
LLLT devices fall into specific classes based on their intended use and associated risks. Understanding these classes can help practitioners choose the right device for their patients:
Practitioners should be well-informed about these classifications to make educated decisions regarding device selection.
The 510(k) process allows manufacturers to demonstrate that their LLLT devices are substantially equivalent to existing products on the market. This pathway helps streamline the approval process while ensuring safety and efficacy. Key points of the 510(k) process include:
This process not only supports innovation but also reinforces patient safety! Further details on the 510(k) submission process for photobiomodulation (PBM) devices can be found in the FDA's guidance documents.
FDA guidelines for medical devices are comprehensive and designed to protect patients. They encompass everything from manufacturing practices to post-market surveillance. By adhering to these guidelines, manufacturers can ensure their LLLT devices are safe and effective for patient use. This commitment to safety is crucial for building confidence among patients and healthcare providers.
As we move forward in our exploration of LLLT, understanding the significance of FDA clearances will help us appreciate the role of these devices in enhancing patient care. Stay tuned for more insights into the safety and efficacy of FDA-cleared LLLT devices!
When considering low-level laser therapy (LLLT), always ask your healthcare provider about the FDA clearance status of the device being used. This not only ensures that you are receiving a safe and effective treatment but also empowers you to make informed decisions about your health.
When it comes to low-level laser therapy (LLLT), the FDA clearance of devices plays a crucial role in shaping patient confidence. Knowing that a device has passed rigorous safety and efficacy evaluations reassures patients that they're making informed choices about their health. This trust in FDA-cleared devices can significantly influence treatment decisions, as patients feel more secure opting for therapies backed by regulatory standards.

Furthermore, FDA clearance often impacts insurance coverage decisions. Many insurance providers recognize these devices as legitimate treatment options, which can help patients manage the cost of their care. In turn, this financial support increases accessibility, helping more individuals benefit from the advantages LLLT offers.
The reliability of LLLT devices is often tied to their FDA clearance. When a device receives this endorsement, it signifies that the manufacturer has met stringent safety guidelines. This validation is not just about regulatory compliance; it's about patient safety and treatment effectiveness. Here are some key points to consider regarding FDA clearance:
For those considering LLLT, understanding these aspects can boost confidence in their treatment options. As a healthcare advocate, I always emphasize the value of choosing FDA-cleared devices—this not only reflects a commitment to quality but also aligns with our mission at Erchonia Laser to provide safe, effective solutions for pain management and overall wellness.
Patients often wonder about the real-world effectiveness of LLLT treatments. While individual experiences may vary, many report positive outcomes when utilizing FDA-cleared devices. It’s essential for patients to understand that results can depend on several factors, including the specific device used, treatment duration, and individual health conditions.
At Erchonia Laser, we believe that education is vital for patients considering LLLT. Understanding FDA documentation can empower individuals to engage in informed discussions with their healthcare providers. Here are some tips for navigating this process:
By fostering awareness of FDA documentation and health insurance coverage, we can help patients make informed decisions about their treatment journeys. After all, empowered patients are better equipped to advocate for their health and well-being!
Here is a quick recap of the important points discussed in the article:
Healing Journeys with Erchonia Laser

Curious about how simple light can transform your pain management journey? Erchonia Laser Therapy of
Understanding Erchonia Laser Therapy Benefits
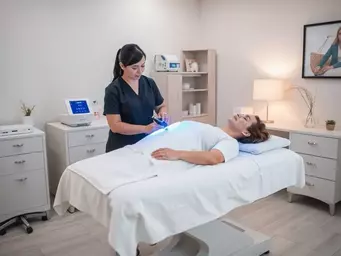
Have you ever considered a solution for chronic pain that doesn’t involve surgery or medication? E
LLLT and Improved Blood Flow
Healing Journeys with Erchonia Laser
Understanding Erchonia Laser Therapy Benefits
Erchonia Devices in Laser Therapy
Biphasic Response in Low-Level Laser