
Erchonia Laser Therapy: FDA Insights
Choosing non-invasive therapies like low-level laser therapy (LLLT) can transform pain management approaches. In a world where effective alternatives are in demand, understanding the science and benefits behind LLLT is crucial for both practitioners and patients. Dive into the essential insights from Erchonia Laser and empower your treatment options.
What You Will Learn
- Low-level laser therapy (LLLT) stimulates cellular activity, enhancing healing and reducing inflammation.
- FDA clearance ensures that Erchonia devices meet rigorous safety and efficacy standards, boosting practitioner and patient confidence.
- LLLT is a non-invasive solution for pain relief, suitable for various conditions with minimal side effects.
- Understanding FDA guidelines for Class II medical devices is crucial for adherence to safety protocols in LLLT applications.
- Erchonia's commitment to education empowers patients and practitioners, promoting informed treatment choices.
- Future research directions aim to expand LLLT applications in diverse fields, enhancing its effectiveness and integration into healthcare.
Erchonia Low-Level Laser Therapy (LLLT) - Key Aspects
The following visual outlines the core components and benefits of Erchonia's FDA-cleared Low-Level Laser Therapy, highlighting its significance and application.
What is LLLT & Erchonia's Role?
Low-level laser therapy uses specific wavelengths of light to stimulate cellular activity, promoting healing and reducing inflammation.
- Stimulates cellular repair
- Promotes non-invasive pain relief
- Supports various clinical recoveries
Importance of FDA Clearance
FDA clearance ensures medical devices meet stringent safety and efficacy standards, providing peace of mind to practitioners and patients.
- Ensures safety compliance
- Instills provider/patient confidence
- Supports treatment credibility
Benefits of Erchonia LLLT
Erchonia LLLT offers a non-invasive approach to pain management, backed by research and rigorous testing.
- Effective pain relief
- Non-invasive & safe
- Versatile applications
Future Directions & Research
Ongoing research aims to expand LLLT applications and enhance effectiveness, driven by evidence-based medicine.
- Expanding clinical trials
- New applications discovery
- Enhanced training programs
Understanding FDA-Cleared Clinical Applications of Erchonia Low-Level Laser Therapy
In the world of pain management, low-level laser therapy (LLLT) has emerged as a powerful tool for both practitioners and patients. At Erchonia Laser, we focus on non-invasive solutions that promote healing and recovery. But how does LLLT fit into the landscape of modern medical treatments? Let's explore this transformative therapy and its significance in clinical applications.
What is Low-Level Laser Therapy (LLLT) and How Does Erchonia Fit In?
Low-level laser therapy utilizes specific wavelengths of light to stimulate cellular activity, enhancing healing and reducing inflammation. At Erchonia, we are dedicated to providing FDA-cleared devices that harness this technology safely and effectively. Our commitment to education and innovation in LLLT helps healthcare professionals better understand this non-invasive option.
- Stimulates cellular repair processes
- Promotes pain relief without invasive procedures
- Supports recovery in various clinical settings
This approach not only empowers patients but also encourages practitioners to explore the benefits of integrating LLLT into their treatment protocols. With a focus on safety and efficacy, Erchonia is paving the way for a brighter future in pain management.
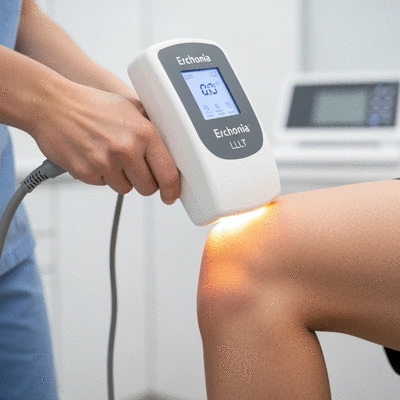
The Significance of FDA Clearance for Erchonia Devices
When considering any medical device, FDA clearance is a crucial factor that ensures safety and efficacy. The devices manufactured by Erchonia have undergone rigorous testing to meet FDA standards, providing practitioners and patients with peace of mind. FDA clearance signifies that our devices have been carefully evaluated and deemed safe for clinical use.
- Ensures compliance with stringent safety regulations
- Instills confidence among healthcare providers and patients
- Supports the credibility of treatment outcomes
In a landscape crowded with various treatment options, having FDA-cleared technology sets Erchonia apart as a trusted leader in the field of LLLT.
Overview of FDA Guidelines for Class II Medical Devices in LLLT
The FDA categorizes devices into different classes, with Class II devices requiring special controls to ensure safety and effectiveness. For LLLT devices, these guidelines include extensive testing for performance and safety. At Erchonia, we adhere to these standards meticulously to provide effective treatment options. You can review an example of such a clearance document for a laser device here.
- Clinical trials to assess safety and effectiveness
- Post-market surveillance for ongoing safety monitoring
- Labeling requirements that inform practitioners and patients
Understanding these guidelines is essential for both practitioners and patients. It reinforces our commitment to advancing evidence-based medicine in low-level laser therapy, thereby creating a more informed approach to pain management.
Pro Tip
To maximize the benefits of low-level laser therapy (LLLT), consider combining it with other non-invasive treatments such as physical therapy or acupuncture. This integrated approach can enhance healing, improve pain management, and promote overall wellness, helping you achieve optimal results in your recovery journey.
Summarizing the Benefits of Erchonia Low-Level Laser Therapy
At Erchonia Laser, we are excited to share the remarkable benefits of low-level laser therapy (LLLT). This non-invasive treatment modality is not just about alleviating pain; it promotes overall wellness by addressing various underlying conditions. Patients and practitioners alike have reported significant improvements in pain management, recovery times, and overall quality of life. But why should you consider Erchonia LLLT specifically for pain relief? Let’s explore the reasons!
- Effective Pain Relief: Many patients experience a noticeable reduction in pain after just a few sessions.
- Non-Invasive Solution: LLLT does not involve needles, surgery, or extensive recovery times.
- Versatile Applications: From post-surgical pain to chronic conditions, LLLT can address a wide range of issues.
- Safe and Well-Tolerated: Minimal side effects make it a suitable option for various patient populations.
As we continue to gather clinical evidence and success stories, the potential for LLLT to transform pain management becomes even clearer. I believe that every patient deserves access to effective, safe, and innovative treatments. That’s why I advocate for LLLT as a key component of pain management strategies.
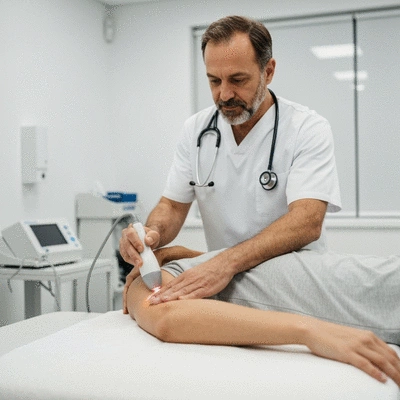
Why Consider Erchonia LLLT for Pain Relief?
Choosing Erchonia LLLT means opting for a therapy that has been rigorously tested and approved for clinical use. Our devices have received FDA clearance, which underscores their safety and efficacy. But what truly sets our approach apart is the commitment we make to educate both patients and practitioners about how LLLT works. By understanding the mechanism behind this therapy, patients can feel empowered and confident in their treatment choice.
- Education and Support: We provide resources that help patients understand the science behind LLLT.
- Tailored Treatment Plans: Each patient's journey is unique, allowing for personalized therapy approaches.
- Proven Technology: Our LLLT devices are backed by extensive research and clinical trials, enhancing trust in their use.
Our clinical experiences have shown that patients who actively participate in their treatment journey often achieve better outcomes. So, it's crucial to have open conversations about expectations and treatment goals.
Frequently Asked Questions About Erchonia Low-Level Laser Therapy
- Q1: What is Low-Level Laser Therapy (LLLT)?
- A1: LLLT uses specific wavelengths of light to stimulate cellular activity, promoting healing and reducing inflammation without invasive procedures.
- Q2: Why is FDA clearance important for Erchonia LLLT devices?
- A2: FDA clearance ensures that Erchonia devices meet stringent safety and efficacy standards, providing confidence to both practitioners and patients in their clinical use.
- Q3: What are the main benefits of Erchonia LLLT?
- A3: Benefits include effective pain relief, a non-invasive treatment approach, versatile applications for various conditions, and minimal side effects, making it a safe and well-tolerated option.
- Q4: How does Erchonia contribute to the advancement of LLLT?
- A4: Erchonia is committed to ongoing research, expanding clinical trials (such as the one detailed on ClinicalTrials.gov), discovering new applications, and providing enhanced training programs for healthcare professionals to maximize LLLT's benefits.
- Q5: What role does evidence-based medicine play in LLLT adoption?
- A5: Evidence-based medicine is crucial for LLLT adoption as it integrates robust data and clinical findings into treatment protocols, fostering trust, and encouraging wider acceptance among healthcare providers.
Future Directions for Research and Clinical Application
The future of Erchonia LLLT holds exciting possibilities! Ongoing research is paving the way for new applications and enhanced effectiveness. We are dedicated to exploring how LLLT can be integrated into various healthcare practices to optimize patient outcomes. Imagine a world where pain management is achieved without invasive procedures—this is the vision we strive for at Erchonia Laser!
- Expanding Clinical Trials: Continued research will validate LLLT’s effectiveness across more conditions, with ongoing studies like the one registered on ClinicalTrials.gov exploring new applications.
- New Applications: Discovering innovative uses for LLLT in fields like sports medicine and rehabilitation.
- Enhanced Training Programs: Equipping practitioners with the knowledge to maximize LLLT benefits for their patients.
As we look ahead, I encourage you to stay tuned for updates. Together, we can push the boundaries of what’s possible with low-level laser therapy!
The Role of Evidence-Based Medicine in Advancing LLLT Adoption
Evidence-based medicine is the cornerstone of our mission at Erchonia Laser. By integrating robust data and clinical findings into our treatment protocols, we ensure that patients receive care that is not only effective but also grounded in scientific research. This dedication fosters trust and encourages wider adoption of LLLT among healthcare providers.
- Supporting Research: We are committed to publishing and sharing research findings that reinforce the validity of LLLT.
- Building Partnerships: Collaborating with medical institutions to further explore LLLT’s impact on pain management.
- Training Healthcare Professionals: Offering resources and training to ensure practitioners are well-informed.
In this rapidly evolving field, staying informed and adapting to new findings is crucial. By embracing evidence-based practices, we not only enhance patient care but also contribute to the growing body of knowledge surrounding LLLT. I invite you to join us on this journey toward a future where pain relief is accessible, effective, and grounded in scientific evidence!
Recap of Key Points
Here is a quick recap of the important points discussed in the article:
- Low-Level Laser Therapy (LLLT): A non-invasive treatment that stimulates cellular activity to promote healing and reduce inflammation.
- FDA Clearance: Erchonia devices are FDA-cleared, ensuring their safety and efficacy for clinical use.
- Clinical Guidelines: Adhering to FDA guidelines ensures that LLLT devices undergo rigorous testing for performance and safety.
- Effective Pain Management: Many patients experience significant pain relief with minimal side effects.
- Education and Support: Erchonia provides resources to help both practitioners and patients understand the benefits of LLLT.
- Future Research: Ongoing studies aim to expand the applications of LLLT in various clinical settings.



LLLT and Improved Blood Flow
Healing Journeys with Erchonia Laser
Understanding Erchonia Laser Therapy Benefits
Erchonia Devices in Laser Therapy
Biphasic Response in Low-Level Laser